News & Blogs 2024-03-25
Key Ingredient for Thyroid Function Tests - Thyroid stimulating hormone
EN
CN
EN
News & Blogs 2024-03-25
Key Ingredient for Thyroid Function Tests - Thyroid stimulating hormone

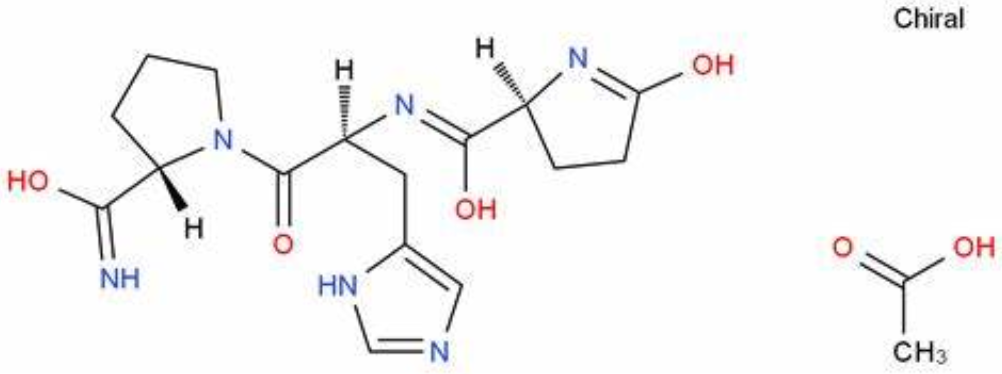
Photo from https://china.guidechem.com/407983/detail.html
Thyroid stimulating hormone (TSH) is a glycoprotein secreted by the anterior pituitary gland, consisting of approximately 211 amino acids. It comprises two peptide chains, α and β, where the β chain only exhibits biological activity when non-covalently bound to the α chain, ensuring the specificity of TSH.
TSH plays a crucial role in regulating thyroid function and serves as a sensitive indicator of the hypothalamic-pituitary-thyroid axis function. [1]
Neurons in the hypothalamus release thyrotropin-releasing hormone (TRH), which stimulates the synthesis and release of TSH from the pituitary gland. TSH is influenced both by the stimulatory effect of TRH and by the feedback regulation of triiodothyronine (T3) and thyroxine (T4) levels in the blood, with both factors antagonizing each other. [1]
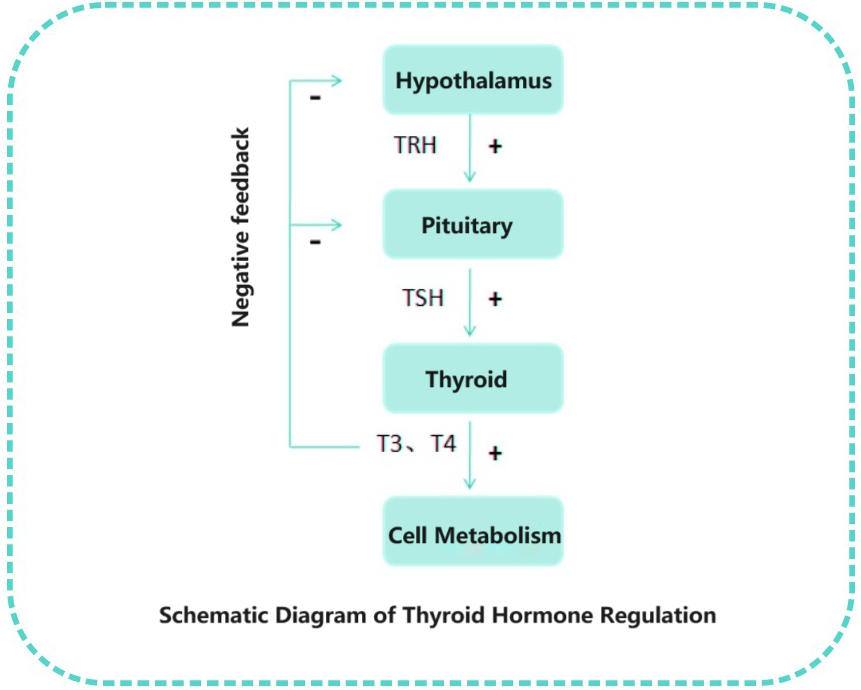
The primary function of thyroid hormones in the human body is to regulate metabolism, promote growth and development, and maintain the balance of metabolic substances such as sugar, fat, and protein. TSH measurement in clinical practice is essential for screening thyroid diseases, diagnosing subclinical thyroid diseases, monitoring replacement therapy for primary hypothyroidism, and assessing conditions such as thyroid nodules, thyroiditis, and thyroid cancer.[2]
Elevated TSH levels indicate thyroid hypofunction, where the thyroid gland fails to produce sufficient hormones, leading to compensatory increased TSH production by the pituitary gland.[1,2] Symptoms of hypothyroidism include fatigue, weight gain, dry skin, and irregular menstruation. Conversely, decreased TSH levels indicate thyroid hyperfunction, where the thyroid gland secretes excessive hormones, resulting in reduced TSH production by the pituitary gland. Symptoms of hyperthyroidism include palpitations, weight loss, and irritability.
Women of childbearing age are at high risk of thyroid diseases, and thyroid diseases during pregnancy mainly include hypothyroidism, hyperthyroidism, thyroid tumors, thyroid nodules, and goiters. Both clinical and subclinical thyroid dysfunction are high-risk factors during pregnancy; therefore, timely screening for thyroid function during pregnancy is essential. In early pregnancy, human chorionic gonadotropin (hCG) increases, and the α subunit of hCG is similar to TSH, possessing a stimulating effect on the thyroid gland. Elevated levels of thyroid hormones suppress TSH secretion.
Hence, during pregnancy, TSH levels decrease by an average of 0.4 mU/L compared to non-pregnant women, and 20% of pregnant women may drop to 0.1 mU/L. The increase in serum hCG levels and the decrease in TSH levels occur between weeks 8-14 of pregnancy, with the lowest point for TSH decline observed during weeks 10-12.[3]
Conclusion , TSH levels are closely related to thyroid function, and both high and low levels can lead to varying degrees of health problems, affecting people's normal lives. Therefore, conducting TSH level testing is essential.
For thyroid stimulating hormone detection, Bioeast offers the following TSH monoclonal antibodies, with recommended antibody pairings as follows:
| PRODUCT NAME | CAT NO. | Type | Application Platform | Pair Recommendation |
| Thyroid Stimulating Hormone (TSH) | TSH101 | Antibody | LF,CLIA,EIA | Coating |
| TSH102 | Antibody | LF,CLIA,EIA | Conjugate |
Recommended pairings for developing sandwich immunoassay detection systems for thyroid stimulating hormone.
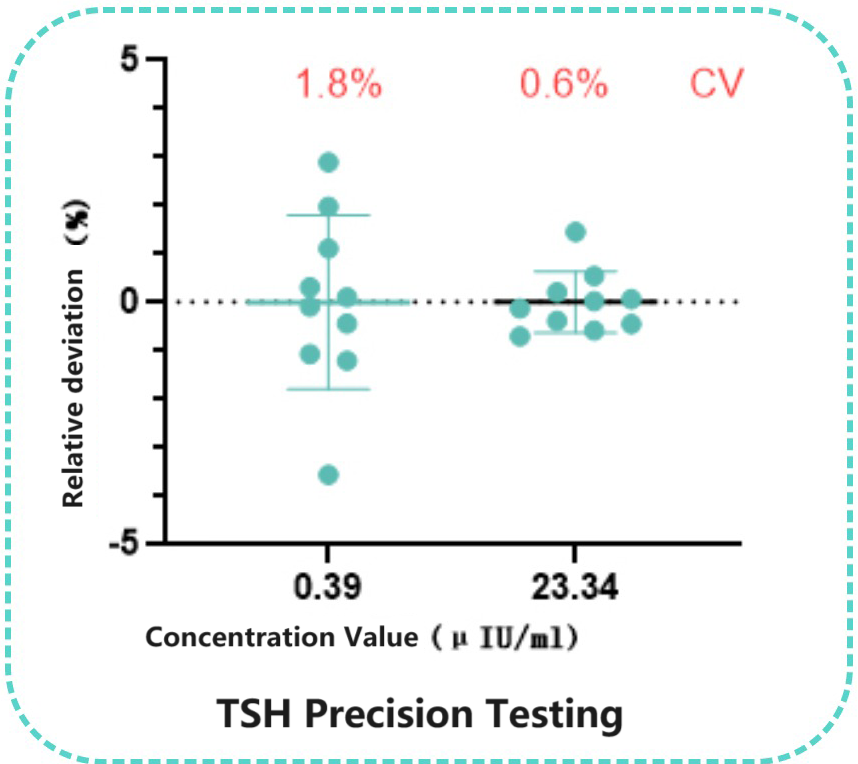
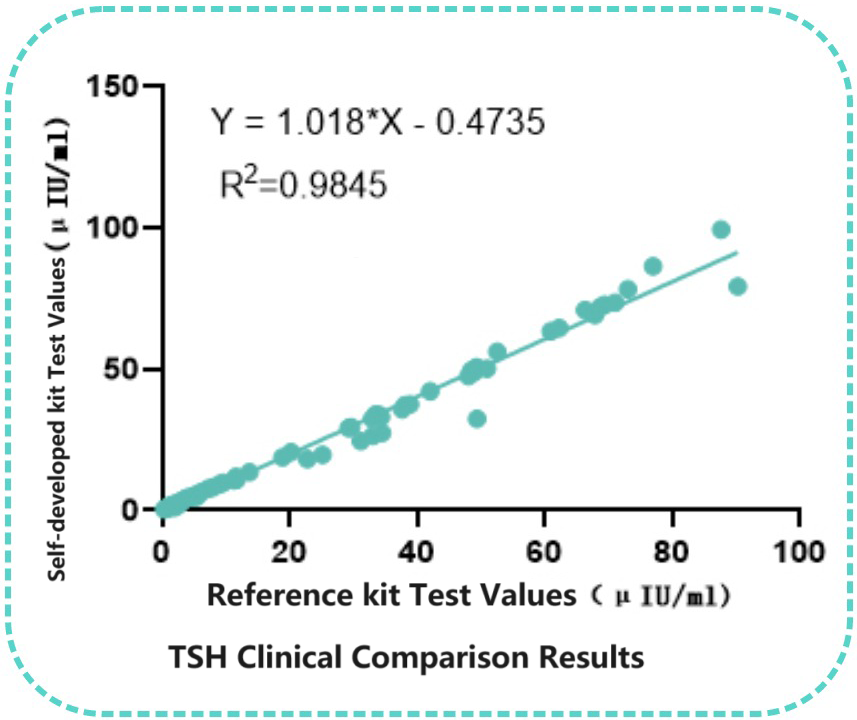
Taking the chemiluminescence platform as an example, Bioeast's internal laboratory selected a pair of TSH monoclonal antibodies for assay performance analysis, with the capture antibody chosen as SH101 and the labeled antibody as TSH 102. The results showed excellent performance of this pairing on the chemiluminescence platform, with precision at the low end ≤5.0%. Additionally, a comparison with 98 clinical samples demonstrated good correlation with the Reference kit(R²=0.9845).
Two clinical samples were tested ten times using the self-developed kit, showing good precision at the low end(CV≤5.0%).
Correlation with Reference kit
Ninety-eight clinical samples were tested using both the self-developed kit and the Reference kit. Linear regression analysis of the concentrations measured by the two kits showed significant correlation (R2=0.9845).
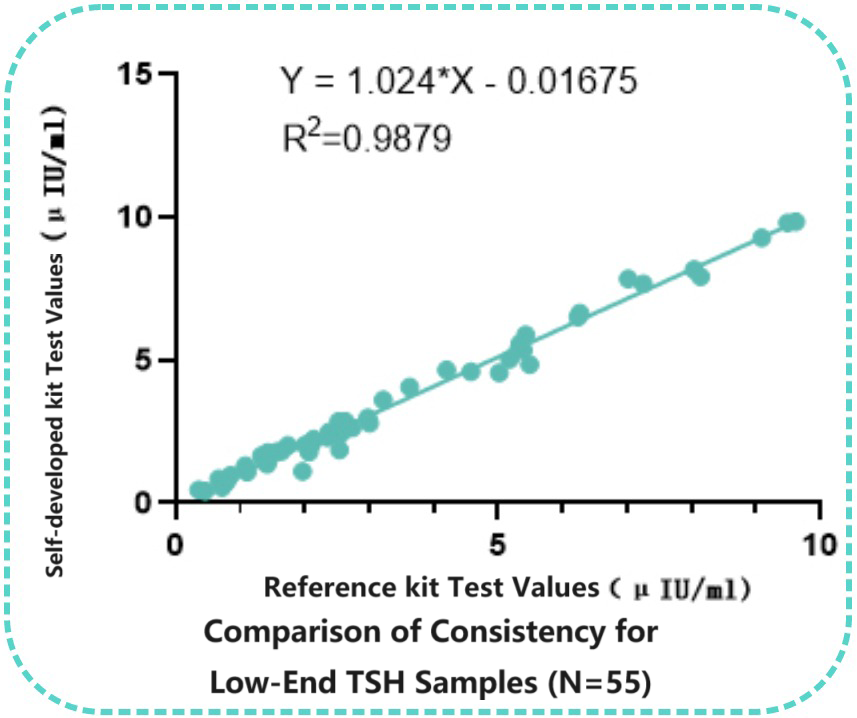
Consistency with Reference kit for Low-End Samples
Fifty-five low-end clinical samples (<10 uIU/ml) were tested in parallel using both the Reference kit and the self-developed kit. Linear regression analysis of the concentrations measured by the two kits demonstrated significant correlation(R2=0.9879).